Interview with Dr. Ann Eshenaur Spolarich
Dr. Ann Eshenaur Spolarich is an internationally recognized author and speaker on pharmacology and the care of … Watch Video
Research suggests that the combination of salivary biomarkers and point-of-care diagnostics could be the answer to predicting periodontal disease in at risk patients.
September 20, 2017
Periodontitis is the diagnosis that about half of all Americans over the age of 30 receive from their dentists.1 As one of the most frequently occurring oral infectious diseases and a leading cause of tooth loss in adults, periodontitis is bacteria-induced site-specific, predominantly chronic, slowly progressing inflammatory disease that affects the supporting structures of teeth, typically alternates between phases of active inflammation and remission, and generally cannot be reversed.2 Similar in nature is peri-implantitis, another adverse condition of the oral cavity, characterized by tissue destruction and inflammation of prosthetic dental implants3; it is recognized a major reason for implant failure.4
Based on decades of research conducted on periodontitis and peri-implantitis, there is much that we are certain of in terms of disease initiation and progression. For instance, evidence supports that periodontal disease can in fact be prevented in many patients. Moreover, we also know that prevention is twofold, meaning that both the dental professional and patient have responsibility in affecting the disease course.5 If we know periodontitis can be prevented, how can we begin to monitor and treat the disease once it has already manifested? The answer: advanced rapid point-of-care (POC) diagnostic tools that use saliva to reveal specifics of the individual patient’s disease state is one of many emerging technologies for disease diagnosis at the early stages.
The role of saliva as a POC diagnostic technique
Currently, the standard route to diagnosis of periodontal disease involves measuring periodontal pocket depth and associated bone loss by manual probing of the gingival sulcus (the natural crevice located between the tooth and gum tissue), visual examination, and two-dimensional radiographic imaging. However, these methods are beginning to become quite antiquated as they are over a century old and with limited information on disease classification, treatment planning, and prognosis.3
We need more precise determinants of periodontal disease in patients, and saliva-based techniques show promise to be just that for many of individuals at risk of disease.
A composite secretory fluid consisting of trace metals, metabolites, biochemicals, proteins, glycoproteins, lipids, and other matter, saliva is imperative to oral and complete body health. Not only does it lubricate surrounding tissues, it also provides tooth mineralizing factors, acid buffers, toxin neutralizers, and antimicrobial components. Though, for the purpose of this article, what is most significant to acknowledge is saliva’s potential ability to serve as a reservoir of biological mediators associated with local oral and systemic conditions, which is how it can help diagnose periodontal disease.6
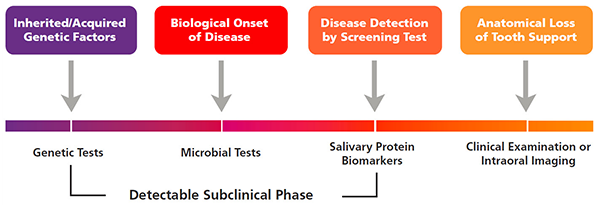
Figure 1. Timeline of periodontal disease progression.
Photo credit: Salivary diagnostics for periodontal diseases, William V. Giannobile, JADA 2012;143(suppl 10):6S-11S
Promising salivary diagnostic tools—that may exist in the near future—would be POC devices found chairside or in a laboratory setting that has the ability to analyze a patient’s salivary fluid sample and supply the dental professional with a quick result. The findings would then be explained to the patient, who would benefit from having an understanding of his or her oral health. This type of diagnostic solution could reveal various factors, such as the patient’s susceptibility to periodontal disease via a genetic test, or even an instant evaluation of the disease progression by means of assessing distinct biological mediators (biomarkers) within the body.7
Salivary biomarkers for the diagnosis of periodontal disease
By virtue of science and research, there is much we know about periodontal disease. For example, in addition to the validity of periodontitis and peri-implantitis being preventable in some cases, the oral health community also knows that there is a variety of salivary biomarkers that can be used to predict disease progression.2 Now, despite there not being a solitary biomarker as of yet, we are aware of several combinations that cater to the multidimensional pathology of the disease and help to identify the patient’s oral health status of his/her dentition.7
Matrix-related mediators
There has been much data collected and many studies performed over the years to determine which biological factors could aid in the diagnosis of periodontal disease; one of these factors in particular being matrix metalloproteinase (MMPs). Found in saliva, MMPs are collagen destroying-enzymes3 that are released during the disease process and damage connective tissue and alveolar bone.8 Exclusive to periodontal disease are MMP-2, MMP-8, MMP-9, and MMP-13 with MMP-8 and MMP-9 (during active periods of the disease) being most central, as they have been proven to be top salivary biomarkers for diagnosis and prognosis of periodontitis.3
Although not widely received, MMPs are often used as diagnostic tools. In 2010, an oral test became available and has shown reasonable sensitivity and specificity in diagnosing periodontal lesions in smoking and nonsmoking patients.7
Inflammatory mediators
Found in oral fluids, inflammatory mediators are a new and readily available benchmark for the diagnosis of periodontal disease and evaluation of overall oral health status.9
Along with the MMP enzymes, there are several inflammatory cytokines (proteins) associated with diagnosis; they include interleukin (IL) 1β, IL-6, TNF-α, 8 and IL-17.7 During the pathogenesis of periodontal disease, these small proteins are released, activating tissue destruction.
With research that caters to the connection between genetics and periodontitis, it is important to note that the IL-1 family, specifically the IL-1 gene is linked to periodontal disease. At this time, there are several prognostic tests (e.g., a saliva-saline matrix oral rinse sample) that can be used to help predict a patient’s periodontal risk when combined with other systemic factors such as smoking or diabetes.10
Microbial mediators
Periodontitis and peri-implantitis are complex diseases that develop differently in each patient. Given that the infections are also local in nature, and as a result saliva becomes an optimal fluid to use for assessment of microbial biomarkers that determine disease progression.7
These biomarkers are presented in the form of pathogens and viruses (although viruses are not typically related to periodontal disease) that can be detected in the teeth’s biofilm. Treponema denticola, Tannerella forsythia, and Prevotella intermedia and Epstein-Barr virus frequently appear in the saliva of patients with progressive periodontitis.7
Although biomarkers for periodontal disease have been extensively researched, they are not yet universally accepted for application to clinical practice because since while early stage clinical studies are promising, there have not been definitive diagnostic trials performed that can demonstrate widespread use clinically.3 The use of combinations of biomarkers such as genetic, microbial, and proteins may offer potential in the development of “signatures” of disease classification for patients.8
The future of salivary diagnostics
When salivary diagnostic POC devices become standard for predicting periodontitis, there is good potential that it will be a pivotal moment for the field and the oral health community in general. Not only will these tools optimize therapy and risk assessment with personalized (precision) dental medicine, they will also likely open the door for expanded healthcare services that will impact patients on a global level.
Acknowledgement
The author thanks Lanmark360 who assisted with the manuscript preparation. The author has previously served as a consultant for Colgate. Dr. Giannobile’s saliva diagnostics technology is licensed to MicroSystemic Healthcare.
William Giannobile is the Najjar Endowed Professor of Dentistry and Chair of the Department of Periodontics and Oral Medicine at the University of Michigan School of Dentistry. He also serves as a Professor of Biomedical Engineering at the College of Engineering, University of Michigan. He has published and lectured extensively on the topic of saliva diagnostics and personalized healthcare. He also practices periodontics and implant dentistry in Ann Arbor, Michigan.
References: